Embark on an illuminating journey with our comprehensive Chemistry in Biology Chapter 6 Answer Key, a meticulously crafted resource that unravels the intricate tapestry of life’s molecular underpinnings. Dive into the fundamental principles of chemistry that orchestrate biological processes, unlocking the secrets of biomolecules, cellular metabolism, and the very essence of life itself.
Our exploration begins with the remarkable properties of water, the lifeblood of biological systems, and delves into the intricacies of pH and its profound impact on cellular harmony. We will decipher the structures and functions of carbohydrates, lipids, proteins, and nucleic acids, unraveling their roles in energy storage, membrane formation, enzyme catalysis, and the storage and transmission of genetic information.
Chemistry of Life
Chemistry is the study of matter and its interactions. Biological chemistry, also known as biochemistry, is the study of the chemical processes that occur in living organisms. These processes include the formation and breakdown of molecules, the transfer of energy, and the regulation of cellular activities.
The fundamental principles of chemistry underpin all biological processes.
Chemical Bonds
Chemical bonds are the forces that hold atoms together to form molecules. The four main types of chemical bonds are covalent bonds, ionic bonds, hydrogen bonds, and van der Waals forces. Covalent bonds are formed when two atoms share electrons.
Ionic bonds are formed when one atom transfers electrons to another atom. Hydrogen bonds are formed when a hydrogen atom is bonded to two electronegative atoms. Van der Waals forces are weak forces that occur between all atoms and molecules.
Biomolecules
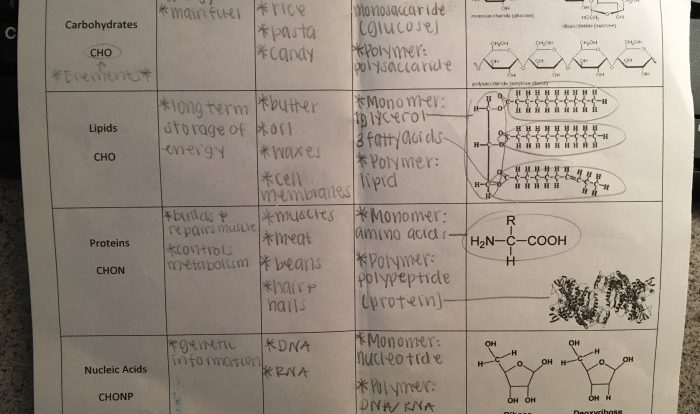
Biomolecules are the molecules that make up living organisms. The four main types of biomolecules are carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates are composed of carbon, hydrogen, and oxygen atoms. Lipids are composed of carbon, hydrogen, and oxygen atoms, and they also contain fatty acids.
Proteins are composed of carbon, hydrogen, oxygen, nitrogen, and sulfur atoms. Nucleic acids are composed of carbon, hydrogen, oxygen, nitrogen, and phosphorus atoms.
Water and pH
Water is the most abundant molecule in living organisms. It is essential for life because it provides a medium for chemical reactions, it helps to regulate body temperature, and it transports nutrients and waste products. The pH of a solution is a measure of its acidity or alkalinity.
The pH scale ranges from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are acidic, and solutions with a pH above 7 are alkaline.
Importance of Water
Water is important for life because it provides a medium for chemical reactions. Water molecules are polar, meaning that they have a positive end and a negative end. This polarity allows water molecules to dissolve ionic compounds and polar molecules.
Water also helps to regulate body temperature. When water evaporates, it takes heat away from the body. This helps to keep the body cool.
pH and Enzyme Activity
The pH of a solution can affect the activity of enzymes. Enzymes are proteins that catalyze chemical reactions. Enzymes work best at a specific pH. If the pH of the solution is too low or too high, the enzyme will not be able to function properly.
Carbohydrates
Carbohydrates are composed of carbon, hydrogen, and oxygen atoms. They are classified into three main types: monosaccharides, disaccharides, and polysaccharides. Monosaccharides are the simplest carbohydrates. They contain only one sugar unit. Disaccharides are composed of two monosaccharides.
Polysaccharides are composed of many monosaccharides.
Energy Storage
Carbohydrates are the body’s main source of energy. When carbohydrates are broken down, they release energy that can be used to power the body’s cells.
Structural Significance, Chemistry in biology chapter 6 answer key
Polysaccharides also have structural significance. For example, cellulose is a polysaccharide that is found in the cell walls of plants. Cellulose provides strength and support to the cell wall.
Lipids
Lipids are composed of carbon, hydrogen, and oxygen atoms. They are classified into four main types: fats, oils, phospholipids, and steroids. Fats and oils are composed of fatty acids and glycerol. Phospholipids are composed of fatty acids, glycerol, and a phosphate group.
Steroids are composed of four fused rings of carbon atoms.
Cellular Membranes
Lipids are the main components of cellular membranes. Cellular membranes are the barriers that surround cells and protect them from their surroundings. Lipids are also involved in energy storage and signaling.
Cholesterol
Cholesterol is a type of steroid that is found in the cell membranes of animals. Cholesterol is essential for membrane fluidity and hormone synthesis.
Proteins

Proteins are composed of carbon, hydrogen, oxygen, nitrogen, and sulfur atoms. They are composed of amino acids, which are linked together by peptide bonds. Proteins are classified into two main types: globular proteins and fibrous proteins. Globular proteins are spherical in shape and they are soluble in water.
Fibrous proteins are long and thin and they are insoluble in water.
Enzyme Catalysis
Proteins are involved in a wide range of cellular processes, including enzyme catalysis, immune response, and cell signaling. Enzymes are proteins that catalyze chemical reactions. Enzymes speed up the rate of chemical reactions by lowering the activation energy of the reaction.
Nucleic Acids
Nucleic acids are composed of carbon, hydrogen, oxygen, nitrogen, and phosphorus atoms. They are composed of nucleotides, which are linked together by phosphodiester bonds. There are two main types of nucleic acids: DNA and RNA. DNA is the genetic material of cells.
RNA is involved in protein synthesis.
Genetic Information
DNA contains the genetic information that is necessary for the development and function of an organism. DNA is made up of four different nucleotides: adenine, thymine, cytosine, and guanine. The sequence of these nucleotides determines the genetic code.
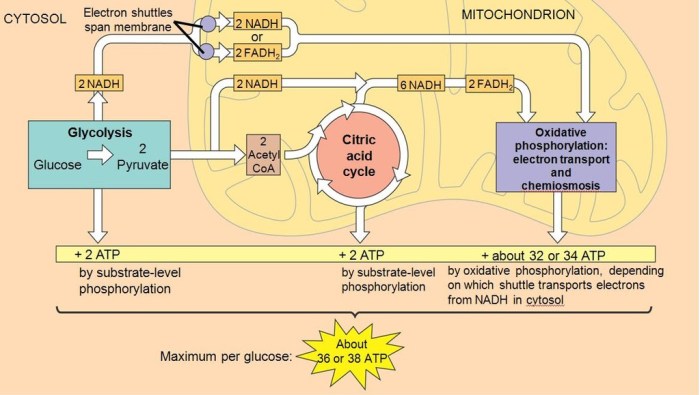
Metabolism
Metabolism is the sum of all chemical reactions that occur in living organisms. Metabolism can be divided into two main types: catabolism and anabolism. Catabolism is the breakdown of complex molecules into simpler molecules. Anabolism is the synthesis of complex molecules from simpler molecules.
Role of Enzymes
Enzymes play a key role in metabolism. Enzymes catalyze the chemical reactions that occur in metabolism. Enzymes speed up the rate of these reactions by lowering the activation energy of the reaction.
Bioenergetics: Chemistry In Biology Chapter 6 Answer Key
Bioenergetics is the study of energy flow in living organisms. Energy is the ability to do work. Living organisms need energy to grow, reproduce, and maintain homeostasis.
ATP
ATP is the universal energy currency in cells. ATP is a small molecule that is composed of adenine, ribose, and three phosphate groups. ATP is used to power the cell’s activities. When ATP is broken down, it releases energy that can be used to drive other cellular processes.
FAQ Summary
What is the significance of chemical bonds in biological systems?
Chemical bonds are the molecular glue that holds biomolecules together, determining their structure and function. They facilitate the formation of complex molecules essential for cellular processes, including enzymes, proteins, and nucleic acids.
How does pH impact cellular processes?
pH plays a crucial role in cellular processes by influencing enzyme activity. Enzymes are highly sensitive to pH changes, and deviations from their optimal pH can disrupt their function, impairing cellular metabolism and homeostasis.
What is the role of carbohydrates in energy storage?
Carbohydrates serve as the primary energy source for living organisms. They are stored in the form of glycogen in animals and starch in plants, providing readily accessible fuel for cellular activities.