The chapter 7 chemical reactions worksheet answer key provides a comprehensive guide to the fundamental concepts of chemical reactions, empowering students with a deep understanding of this essential aspect of chemistry. This key unlocks the answers to complex questions, fostering a solid foundation for further exploration in the field.
Delving into the intricacies of chemical reactions, the worksheet covers a wide range of topics, including key concepts, types of reactions, balancing equations, stoichiometry, reaction rates, and equilibrium. Through engaging examples and detailed explanations, students gain a thorough understanding of the principles governing chemical transformations.
Chapter 7 Chemical Reactions Worksheet Answer Key Overview
A chapter 7 chemical reactions worksheet provides students with practice problems to reinforce the concepts they have learned about chemical reactions. Answer keys are essential for students to check their work and identify areas where they need additional support.
Key Concepts in Chapter 7 Chemical Reactions: Chapter 7 Chemical Reactions Worksheet Answer Key
Chapter 7 of a chemistry textbook typically covers the following key concepts:
- Types of chemical reactions
- Balancing chemical equations
- Stoichiometry
- Reaction rates and equilibrium
The worksheet questions are designed to assess students’ understanding of these concepts.
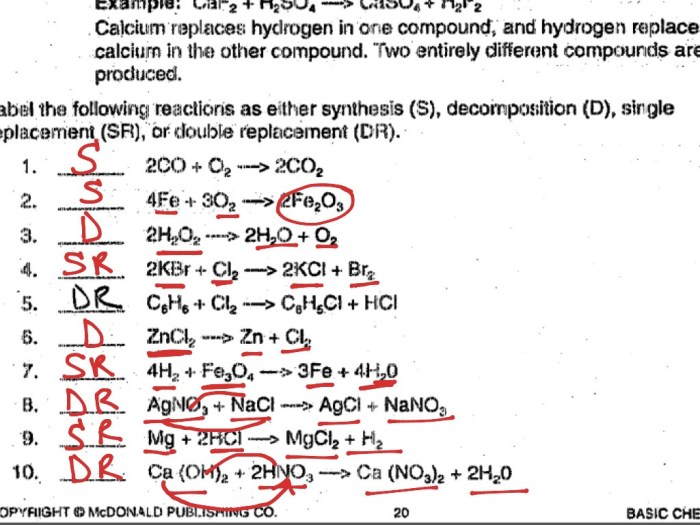
Types of Chemical Reactions
| Reaction Type | Reactants | Products | Balanced Equation |
|---|---|---|---|
| Combination | A + B | AB | A + B → AB |
| Decomposition | AB | A + B | AB → A + B |
| Single Replacement | A + BC | AC + B | A + BC → AC + B |
| Double Replacement | AB + CD | AD + CB | AB + CD → AD + CB |
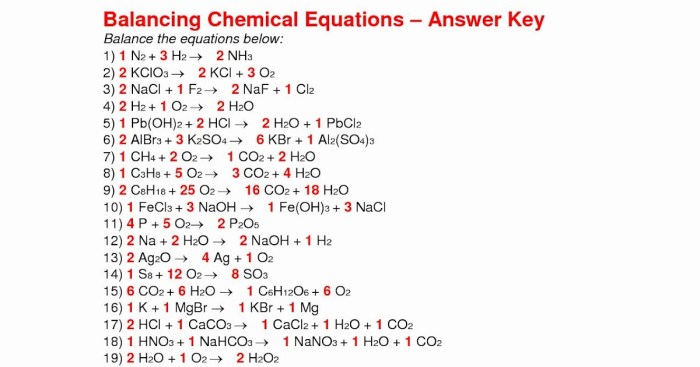
Balancing Chemical Equations
To balance a chemical equation, the number of atoms of each element on the reactants’ side must equal the number of atoms of that element on the products’ side.
For example, the unbalanced equation for the combustion of methane is:
CH4+ 2O 2→ CO 2+ 2H 2O
To balance this equation, we need to add a coefficient of 2 in front of the CO 2and H 2O:
CH4+ 2O 2→ 2CO 2+ 2H 2O
This equation is now balanced because there are equal numbers of atoms of each element on both sides.
Stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in a chemical reaction.
Using stoichiometry, we can calculate the amount of reactants or products that are needed or produced in a reaction.
For example, if we know that the reaction between methane and oxygen produces carbon dioxide and water, we can use stoichiometry to calculate the amount of carbon dioxide that will be produced when we burn a certain amount of methane.
Reaction Rates and Equilibrium
The rate of a chemical reaction is the speed at which the reaction occurs.
There are many factors that can affect the rate of a reaction, including the concentration of the reactants, the temperature, and the presence of a catalyst.
Chemical equilibrium is a state in which the forward and reverse reactions in a chemical reaction are occurring at the same rate.
At equilibrium, the concentrations of the reactants and products do not change over time.
Applications of Chemical Reactions, Chapter 7 chemical reactions worksheet answer key
Chemical reactions are essential for many processes in everyday life, including:
- The combustion of fuels
- The production of food
- The manufacture of medicines
- The cleaning of water
Understanding chemical reactions is essential for a wide variety of fields, including chemistry, biology, and engineering.
Commonly Asked Questions
What is the purpose of a chapter 7 chemical reactions worksheet?
The chapter 7 chemical reactions worksheet is designed to reinforce the concepts of chemical reactions covered in the textbook, providing students with an opportunity to practice and apply their knowledge.
Why is it important to provide answer keys for student learning?
Answer keys are crucial for student learning as they allow students to check their understanding, identify areas for improvement, and gain confidence in their grasp of the subject matter.